Home Prenatal Screening & Diagnostics NICC® (Non-Invasive ChromosomesCheck)
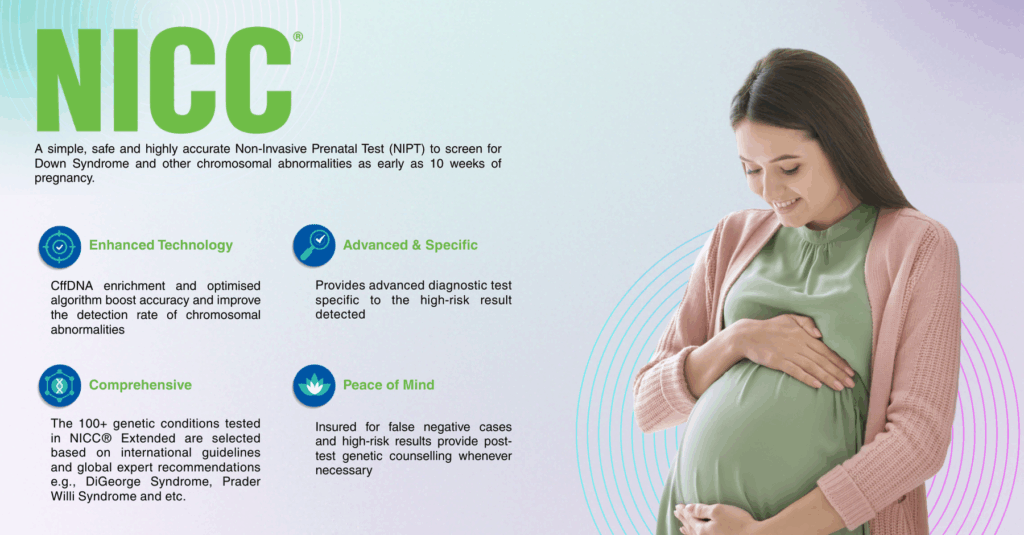
NICC® is a non-invasive prenatal test (NIPT) that uses a small sample of the mother’s blood to assess the likelihood of certain chromosomal conditions in the fetus. It analyzes cell-free fetal DNA present in maternal blood, providing a safe and highly accurate method to screen for common genetic conditions in both singleton and twin pregnancies.
By combining advanced next-generation sequencing (NGS) technology with powerful bioinformatics algorithms, NICC® enables clinicians to offer expecting parents reliable insights into their baby’s genetic health, without any risk to the mother or baby.
risk to the mother or baby.
The NICC® test incorporates a specialized enrichment process to increase the proportion of baby’s DNA in the mother’s blood sample, making it easier to get accurate results from the start. This fetal fraction enrichment process helps deliver:
NICC® can be performed from 10 weeks of pregnancy, with results typically available within 6 to 10 working days after the sample is received by the laboratory.
NICC® is recommended for use in primary screening, particularly in pregnancies with:
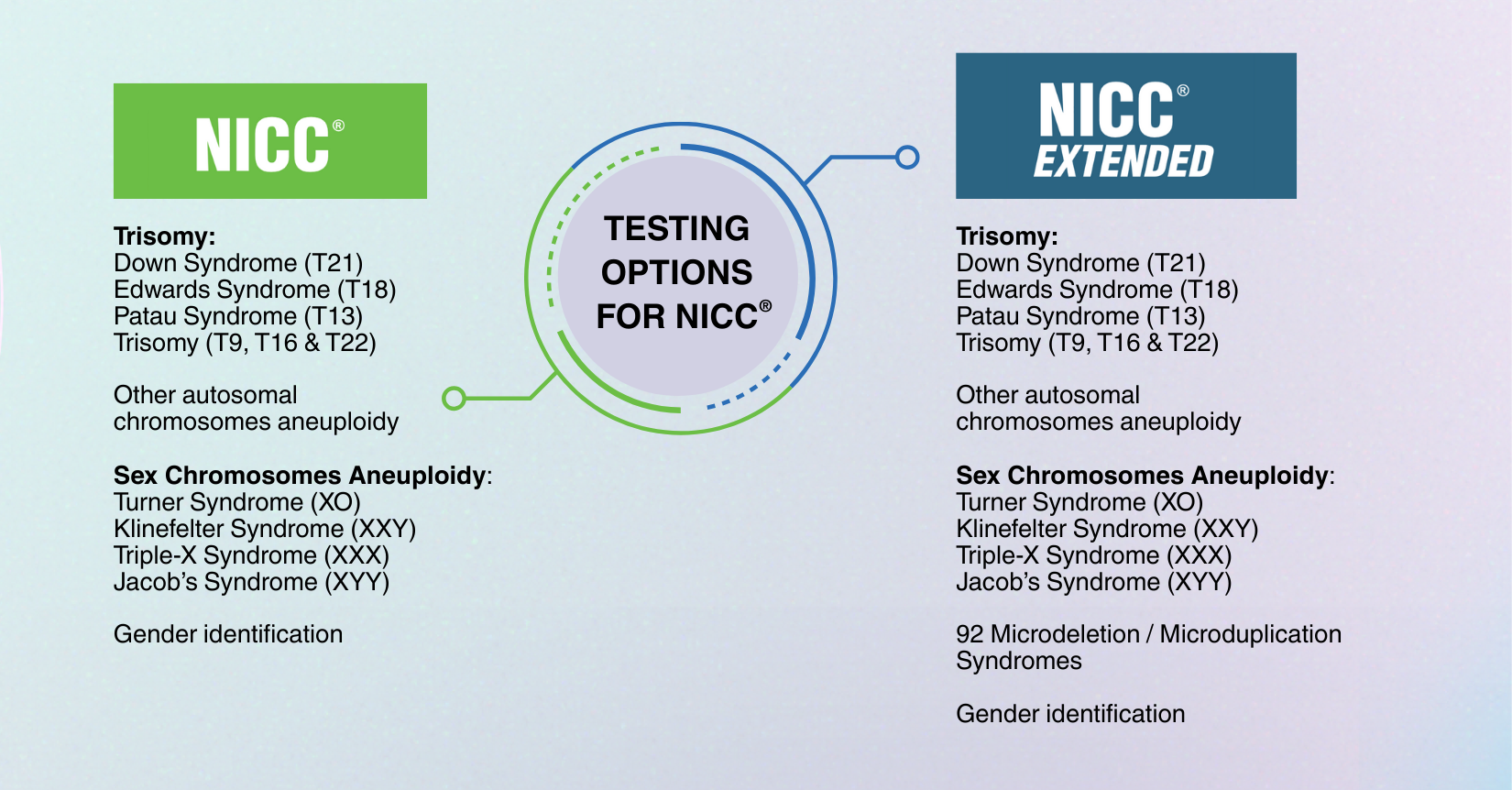
NICC® Extended screens for 92 microdeletion and microduplication syndromes, providing a more comprehensive view of fetal chromosomal health during pregnancy.
Down Syndrome (Trisomy 21) is the most common chromosomal condition, occurring in about 1 in 700 pregnancies worldwide. While standard prenatal screening often includes Down Syndrome, Edwards Syndrome and Patau Syndrome, other important chromosomal changes such as MMSs are much rarer but equally significant. NICC® Extended emphasizes the benefits of broader genetic coverage, ensuring parents and clinicians have access to critical information that supports informed decisions and personalized care.
Many of these changes are not detectable by standard ultrasounds or basic NIPT, making NICC® Extended a valuable tool for early insight.
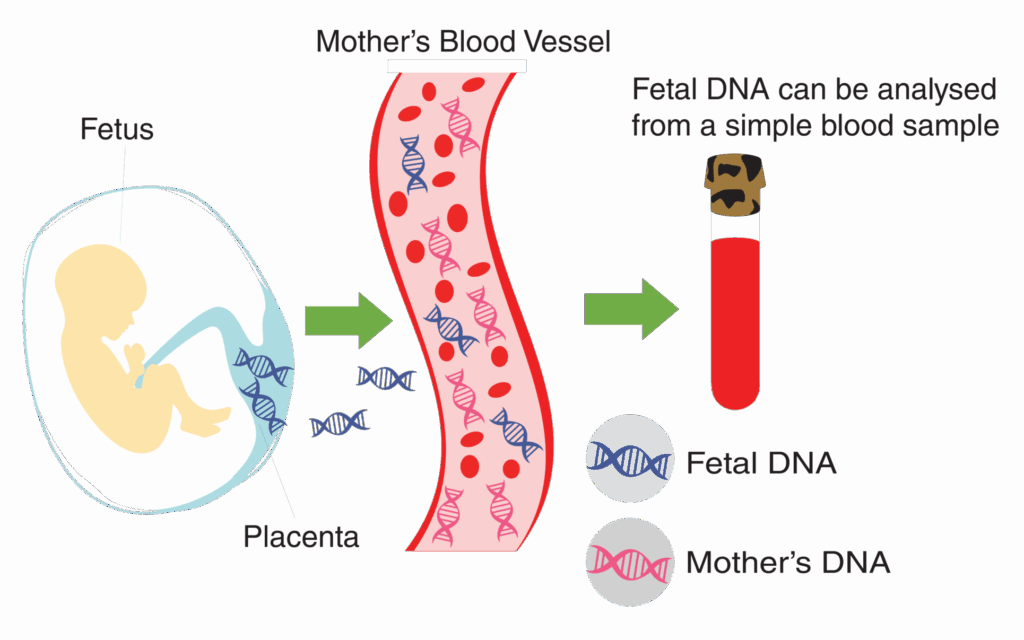
Cell-free fetal DNA (cffDNA) is a tiny fragment of genetic material released from the placenta that circulates in the mother’s blood during pregnancy.
Even in the early weeks of pregnancy, small amounts of cffDNA can be detected, with levels increasing as the pregnancy progresses. Because cffDNA carries the baby’s genetic information, NICC® can detect and analyze it to assess the risk of chromosomal abnormalities.
By identifying potential risks early, NICC® gives parents and healthcare providers valuable time to plan the next steps and make informed decisions, ensuring the best possible care for both mother and baby.

| Specimen Requirements | |
|---|---|
| Clinical usage | Non-invasive prenatal test (NIPT) to assess the risk of fetal chromosomal aneuploidies for: – Trisomies (T21, T18, T13, T9, T16 & T22) – Sex chromosome aneuploidies (XO, XXY, XXX & XYY) – Other autosomal chromosomes aneuploidy – Gender (optional) NOTE: – NICC® test comes with insurance coverage – Sex chromosome aneuploidies testing is not applicable for twin pregnancy |
| Type of sample | 8 – 10 ml maternal blood in Streck® tube |
| Patient requirement | Gestation age: 10 weeks onwards No fasting required IMPORTANT: – Please read and sign the consent on the test request form & medical insurance consent form – In the case of vanishing twin: i. Please ensure to include the date of loss for the vanishing twin ii. ONLY applicable for developmental arrest that occurs before 8 weeks of pregnancy iii. Sampling can ONLY be done at least 8 weeks after the developmental arrest |
| Storage & transportation | Streck® tube must be transported in ROOM TEMPERATURE (20° – 35° C) and must arrive in laboratory within 96 hours after blood drawing. DO NOT REFRIGERATE OR FREEZE THE SAMPLE. |
| Turnaround Time (TAT) | 6 working days |
NICC® test can be done anytime after the 10th week of pregnancy for both singleton and twin pregnancy. This timing ensures there is enough fetal DNA in the mother’s blood for accurate analysis
• In the case of ‘vanishing twin’:
– NICC® only applicable for developmental arrest that occurs before 8 weeks of pregnancy.
– Sampling can only be done at least 8 weeks after the developmental arrest.
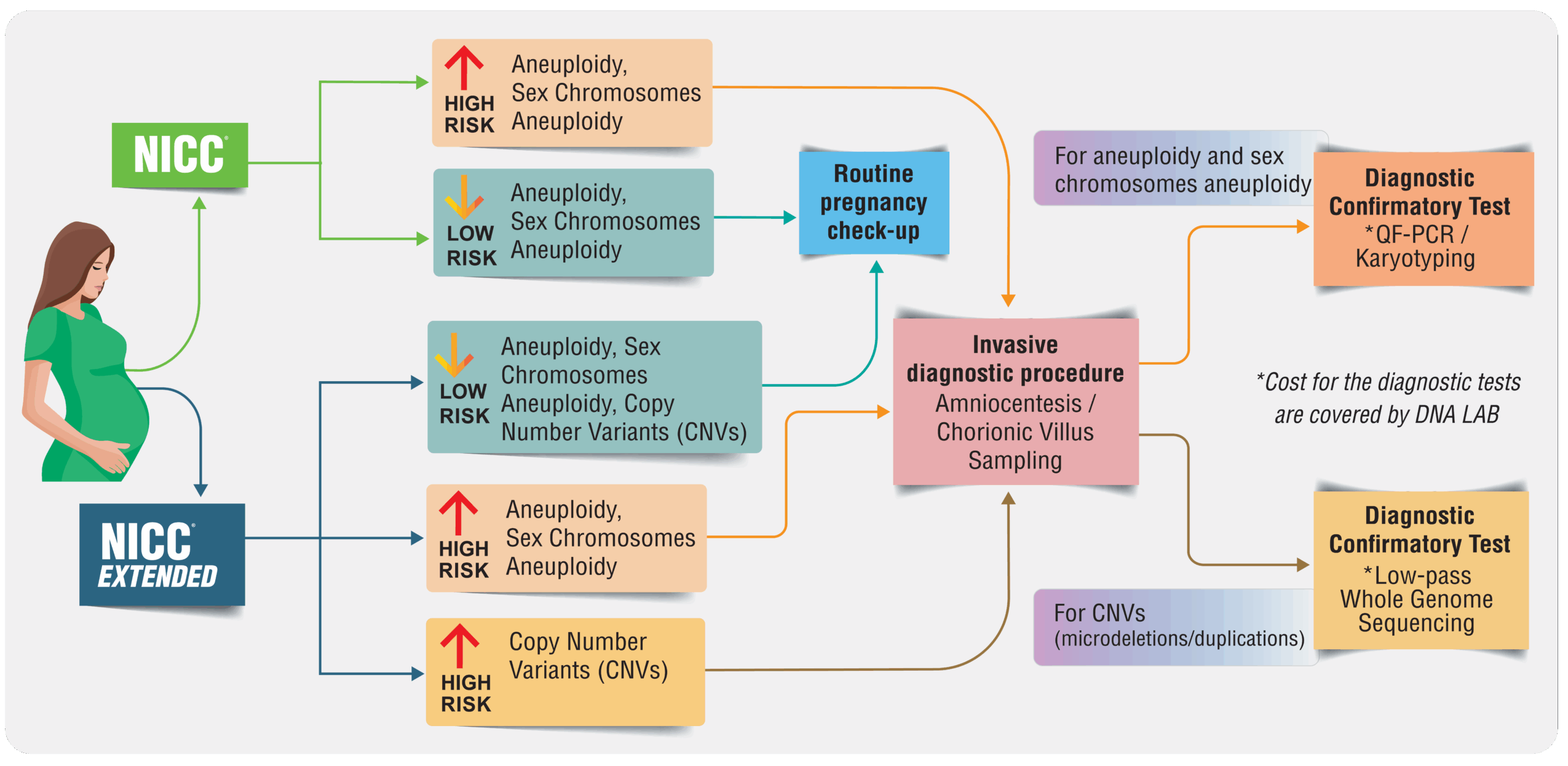
NICC® measures the risk of certain chromosomal abnormalities The test can only estimate whether the risk of having certain conditions is increased or decreased
• Low risk – Means lower risk of developing the tested conditions. Routine follow ups should be performed to monitor the baby’s growth
• High risk – Means higher chance of having chromosomal abnormalities. Diagnostic tests should be performed to confirm the result
While NICC® is a highly accurate and beneficial screening tool for detecting chromosomal abnormalities, it cannot tell for sure whether or not your baby has any of the specific chromosome conditions.
• As a screening test, false positive and false negative result can still occur. The followings may compromise the accuracy of the test:
– Pregnancy with malignant tumor
– Multiple pregnancy, triplets or more
– Received organ transplantation & stem cell therapy
– Genetic abnormalities in any 1 of the parent

Allow us 2-3 days on weekends to tend to your enquiry.
Phone:
+603-7491 1700
Hotline:
+6019-600 2700
Fax:
+603-7499 3997
Our Services
Our Tests
Phone: +603-7491 1700
Hotline: +6019-600 2700
Fax: +603-7499 3997

Monday-Friday
8.30 am – 5.30 pm
Saturday
8.30 am – 1.00 pm